
State of Sanctuary Resources
This section provides summaries of the condition and trends within four resource areas: water, habitat, living resources, and maritime archaeological resources. For each, sanctuary staff and selected outside experts considered a series of questions about each resource area. The set of questions derive from the National Marine Sanctuary System’s mission, and a system-wide monitoring framework (National Marine Sanctuary Program 2004) developed to ensure the timely flow of data and information to those responsible for managing and protecting resources in the ocean and coastal zone, and to those that use, depend on, and study the ecosystems encompassed by the sanctuaries. The questions are meant to set the limits of judgments so that responses can be confined to certain reporting categories that will later be compared among all sanctuary sites and combined. Appendix A (Rating Scheme for System-Wide Monitoring Questions) clarifies the set of questions and presents statements that were used to judge the status and assign a corresponding color code on a scale from “good” to “poor.” These statements are customized for each question. In addition, the following options are available for all questions: “N/A” – the question does not apply; and “undetermined” – resource status is undetermined. In addition, symbols are used to indicate trends: “▲” – conditions appear to be improving; “▬” – conditions do not appear to be changing; “▼” – conditions appear to be declining; and “?” – the trend is undetermined.
This section of the report provides answers to the set of questions. Answers are supported by specific examples of data, investigations, monitoring and observations, and the basis for judgment is provided in the text and summarized in the table for each resource area. Where published or additional information exists, the reader is provided with appropriate references and Web links.
Water
Contaminants may be transported from land across the inner shelf to Gray’s Reef National Marine Sanctuary, but the quantity of material from this process is affected by the trapping efficiency of salt marsh estuaries. The concentration of nutrients in the water not only varies with outwelling events, which are affected by freshwater inputs and oceanographic events, but also with the rates of exchange of contaminants between the water and silt-clay particles in the sediments.
NOAA’s National Ocean Service has conducted sampling along three cross-shelf transects, extending from the mouths of Sapelo, Doboy and Altamaha sounds, and showed a general pattern of decreasing trace concentrations of contaminants with increasing distance from shore, suggesting possible sources from outwelling through coastal sounds. Data also revealed higher percentages of silt-clay fractions in sediments at stations closest to the sounds. These finer-grained particles represent a potential source for adsorption of chemical contaminants entering these systems. Cross-shelf differences in salinity and temperature provided additional evidence of the influence of the sounds, especially the Altamaha, on the adjacent shelf environment. The atmosphere is also considered a pathway of contaminants such as heavy metals, persistent organic contaminants and nutrients to the reef (NMSP 2006, Harris et al. 2004).
1. Are specific or multiple stressors, including changing oceanographic and atmospheric conditions, affecting water quality?
Water quality in the sanctuary is considered to be good based on assessments during spring 2000 and 2005. The trend, however, is undetermined. Unfortunately, there is insufficient information to determine whether changing oceanographic and atmospheric conditions are affecting water quality. In 2005, sanctuary staff in collaboration with the Skidaway Institute of Oceanography developed a more extensive water quality monitoring plan to assess whether trends observable in the coastal region are being reflected in water quality at Gray’s Reef. Measurements include temperature, salinity, dissolved oxygen, inorganic nutrients (NO2/NO3, NH4, PO4, Si(OH)4), organic nutrients (DON, urea, DOC), chlorophyll-a, and a number of bacteriological parameters including total bacteria counts, total and fecal coliforms, enterococci, and the ratio of bioluminescent to total heterotrophic bacteria. Harmful algal bloom species are not currently being examined at the Gray’s Reef sanctuary.
Specific chemical contaminants have not been measured in the water column, but are expected to be very low or undetectable because of the low concentrations found in sediments and biota. In addition, a bacterial indicator of chemical contamination (ratio of bioluminescence to total bacteria; Frischer et al. 2005) suggests an absence of chemical contaminants in the water column at the Gray’s Reef sanctuary (Frischer unpublished data). Dissolved oxygen levels, a primary indicator of water quality, are high throughout the sanctuary. Results of a baseline characterization conducted in 2000 (Hyland et al. 2006, Cooksey et al. 2004) indicated that dissolved oxygen values ranged from 7.6-8.4 mg l-1, which are well above a reported benthic hypoxic effect threshold of about 1.4 mg l-1 (Diaz and Rosenberg 1995) and most state standards of 5 mg l-1 or lower. A follow-up survey conducted in 2005 and ongoing monitoring showed consistent values in this same range (Balthis et al. 2007, Frischer unpublished data). All nutrient, chlorophyll-a, and total bacterial abundance indicate that water quality at Gray’s Reef, in terms of those parameters, is good and not changing.
Currently, anthropogenic stressors that may affect the water quality in the sanctuary — including increasing human activity in the coastal zone — are relatively low. Although some contaminants have been identified in fish and benthic organisms, to date, all have been below EPA guidelines. However, this does not mean that potential problems do not exist. As coastal development and population density continues to increase, offshore water quality will be impacted. This is an area that the sanctuary needs to continuously monitor in order to determine if conditions are changing. In the future, baseline data will help determine whether stressors such as population increases in the coastal zone are influencing water quality at Gray’s Reef.
Changing salinity patterns on the continental shelf off Georgia are also a potential stressor for coastal and shelf species that currently inhabit Gray’s Reef. Natural drought (currently at the highest level of “exceptional” in the southeast U.S.) and increasing human freshwater extraction from dwindling watersheds that feed the coastal zone have had dramatic effects on coastal ecosystems recently (Visser et al. 2002). This freshwater runoff has historically penetrated across the shelf to the edge of the Gulf Stream, and is particularly strong during winter and early spring (Li 2001) when many reef fish spawn (Sedberry et al. 2006). The runoff typically carries nutrients from terrestrial sources to ocean waters that serve as the habitat for very young fish larvae (Atkinson et al. 1978), and reduced runoff could result in poor survival of reef fish larvae on the shelf. In addition, the levels of freshwater runoff can have an effect on overall shelf circulation, and their penetration across the shelf can affect Gulf Stream meanders (Atkinson et al. 1978, Blanton 1981) that influence the kinds of organisms found at Gray’s Reef. Because Gray’s Reef is located within the influence of a massive estuarine/riverine system, it has typically had salinities less than the open ocean, and species typical of coastal and estuarine habitats have occurred here. Changing freshwater runoff may influence the fauna of Gray’s Reef, as oceanic and Gulf Stream species replace those coastal species that are less tolerant of higher salinities.
2. What is the eutrophic condition of sanctuary waters and how is it changing?
At present, eutrophication does not appear to have the potential to negatively affect living resources or habitat quality. The trend, however, has not been determined. There is no evidence of eutrophication or incipient eutrophication at Gray’s Reef National Marine Sanctuary as is occurring in the South Atlantic Bight coastal zone (Verity et al. 2006). This finding is based on low and stable nutrient concentrations, seasonal estimates of chlorophyll-a concentrations, the absence of harmful algal bloom events — with the exception of a subsurface bloom of Phaeocystis globosa in 1999 associated with stratified water (Long et al. in prep) — and high and stable dissolved oxygen concentrations in surface and near-bottom waters.
3. Do sanctuary waters pose risks to human health?
While conditions that have the potential to affect human health may exist at Gray’s Reef, human impacts have not been reported. Furthermore, there is no evidence that the threat is changing. Risks to human health in Gray’s Reef sanctuary have been undergoing assessment based on the use of bacterial indicators of fecal contamination. Indicators have included total and fecal coliform bacteria and enterococci bacteria. All indicators were below detection limits in eight samples collected throughout 2005 (Frischer unpublished data), suggesting minimal risks to human health.
Results of a baseline characterization of benthic communities and sediment quality conducted in 2000 (Hyland et al. 2006, Cooksey et al. 2004) also suggested that chemical contaminants in tissues of target benthic species within the sanctuary were below EPA human health guidelines (where available), based on a limited sample population of 10 fillets of black sea bass and nine arc shell composites. Moderate concentrations of lead, however, just below the EPA Level of Concern value of 3 µg/g dry weight, were found in one fish sample (2.6 µg/g) and one arc shell sample (2.9 µg/g). Also, similar to sediments (see Question 7), tissues of both species contained trace concentrations of man-made pesticides (DDT, chlorpyrifos, dieldrin, lindane, heptachlor epoxide) and other chemical substances associated with human sources (PCBs, PAHs). The fact that immobile organisms like the arcs picked up these contaminants, albeit at low concentrations, provides evidence that such materials have made their way to the offshore sanctuary environment, either by air or cross-shelf transport by water from land. Results of a follow-up monitoring survey conducted in 2005 (Balthis et al. 2007) show a similar persistent trend of low yet detectable levels of chemical contaminants in tissues of these same species. Also, migratory species of fish like king mackerel that are currently under contaminant warnings (i.e., for mercury) are actively fished within sanctuary waters.
4. What are the levels of human activities that may influence water quality and how are they changing?
Because of the remote location of Gray’s Reef National Marine Sanctuary from the coastal zone, human activities that may potentially negatively affect water quality in the sanctuary are believed to be limited. Human activities have increased dramatically along the southeastern coastal zone, but based on chemical contaminant and nutrient concentrations measured in the sanctuary there is no evidence of impact from these sources and no evidence that the trends observed in the coastal zone during the past 20 years (Verity et al. 2006) are mirrored in the sanctuary. However, the continued development of the coastal zone is inevitable, and therefore continued monitoring of the Gray’s Reef sanctuary for evidence of this impact should be a continuing research priority.
The following information provides an assessment by sanctuary staff and the Gray’s Reef Research Advisory Panel of the status and trends pertaining to water quality and its effects on the environment:
Water Status and Trends
Status: Good Good/Fair Fair Fair/Poor Poor Undet.
Trends:
▲ Conditions appear to be improving.
- Conditions do not appear to be changing.
▼ Conditions appear to be declining.
? Undeterminted trend.
N/A Question not applicable.
| # |
Status |
Rating |
Basis For Judgement |
Description of Findings |
| 1. |
Stressors |
?
|
2000 and 2005 monitoring data suggest good water quality, with some contaminants but below EPA guidelines; insufficient information to assess trend |
N/A |
| 2. |
Eutrophic Condition |
?
|
Stable nutrients, chlorophyll, lack of harmful algal blooms |
Conditions do not appear to have the potential to negatively affect living resources or habitat quality.
|
| 3. |
Human Health |

|
2000 baseline, 2005 indicators below EPA Levels of Concern |
Selected conditions that have the potential to affect human health may exist, but human impacts have not been reported.
|
| 4. |
Human Activities |

|
Increasing, but little evidence of negative effects |
Few or no activities occur that are likely to negatively affect water quality.
|
Habitat
Gray's Reef is a submerged hard-bottom (limestone) area that, compared to surrounding areas, contains extensive but discontinuous rock outcroppings of moderate (6-10 feet) height with sandy, flat-bottomed troughs between. The series of rock ledges and sand expanses has produced a complex habitat of caves, burrows, troughs and overhangs that provide a solid base upon which the sanctuary’s abundant sessile invertebrates can attach and grow. This rocky platform, with its carpet of attached organisms, is known as a “live-bottom habitat”. This topography supports an unusual assemblage of temperate and tropical marine flora and fauna. Algae and invertebrates grow on the exposed rock surfaces; dominant invertebrates include sponges, barnacles, sea fans, hard corals, sea stars, crabs, lobsters, snails and shrimps. The reef attracts numerous species of benthic and pelagic fishes, including black sea bass, snapper, groupers and mackerels.
5. What is the abundance and distribution of major habitat types and how is it changing?
Selected habitat loss or alteration has taken place at Gray’s Reef, precluding full development of living resource assemblages, but it is unlikely to cause substantial or persistent degradation in living resources or water quality. The trend is undetermined. The sanctuary completed the first comprehensive habitat classification in 2001 using multibeam and side-scan sonar surveys ground truthed by diver observations and ROV video and still photography (Kendall et al. 2005). The sonar imagery, which completely covers the sanctuary, was mosaiced and georeferenced for use in GIS analysis of bottom type and benthic habitats. This analysis documents the four major habitat types and their spatial extent in the sanctuary: densely colonized live bottom (0.6%), sparsely colonized live bottom (24.8%), rippled sand (66.9%) and flat sand (7.7%) (Figure 22). Previous side-scan surveys of the sanctuary in the 1980s were used to characterize bottom types. Direct comparisons are not straightforward with the new, multiple datasets because of differences in available data types and line spacing. However, efforts to quantify the level of error in older data are ongoing so that decadal changes in habitat distribution can potentially be determined. Preliminary comparisons suggest that areas of low relief in the southeastern quadrant of the sanctuary have been buried by influx of sand on these timescales.
A recent survey of 179 sites within the Gray’s Reef sanctuary indicates that the four bottom types have distinct physical and biological characteristics (Kendall et al. 2007). Sparse live bottom and ledges are colonized by macroalgae and numerous invertebrates, including coral, gorgonians, sponges, tunicates, anemones and bryozoans. Biotic cover on sparse live bottom is less in comparison to ledges, likely because colonization is inhibited by shifting sands. In addition, percent cover of biota on ledges is positively related to ledge height (Kendall et al. 2007). The densely colonized live bottom, although comprising a small percentage of the total sanctuary area, is the critical habitat impacted by pressures and is disproportionate in its importance. Thus, small impacts to a very spatially limited habitat are a particular management concern for this sanctuary. Anthropogenic pressures are not significantly affecting the abundance or distribution of habitat types based on diver observations. Although flat and rippled sand bottom have a low percent cover of epibenthic organisms, these bottom types harbor diverse infaunal assemblages (Hyland et al. 2006).
There is presently an inadequate time series of data with which to determine trends in habitat abundance and distribution. However, the sanctuary now has a comprehensive baseline survey from which future change can be confidently assessed.
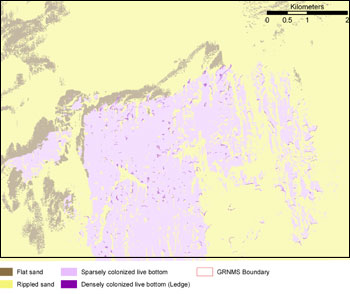 Figure 22. Gray's Reef National Marine Sanctuary Benthic Habitat Map. (Source: Kendall et al. 2005)
Figure 22. Gray's Reef National Marine Sanctuary Benthic Habitat Map. (Source: Kendall et al. 2005)
6. What is the condition of biologically structured habitats and how is it changing?
Currently, there is insufficient information on the complex biological structure of habitats to rate the condition. There is, however, evidence of anchor, fishing and storm damage. The trend is undetermined. Gray’s Reef National Marine Sanctuary is composed of four main bottom types: flat sand, rippled sand, sparsely colonized live bottom and densely colonized live bottom (ledges). Non-quantitative assessments and observations (e.g., dislodgement of sponges, corals and other invertebrates) by scientists, sanctuary staff and users indicates that damage to densely and sparsely colonized live bottom is primarily associated with anchoring. Recreational fishing may also impact biologically structured habitats through marine debris, especially through entanglement in monofilament line (Kendall et al. 2007). Although the impact is minimal, disturbances by divers are also occurring. Damage to biologically structured habitats is disproportionate on a spatial scale and is probably concentrated in areas of highest fishing and diving activity. Recently established long-term monitoring of the benthos indicates that changes in biologically structured habitats at shallow depths also occur due to storm impacts (i.e., movement of sediment) or on seasonal cycles (Freeman et al. 2007). The inability to decipher changes resulting from human impacts versus natural processes makes the trend undetermined at present. Continued monitoring at a range of spatial and temporal scales is required to establish the trend.
7. What are the contaminant concentrations in sanctuary habitats and how are they changing?
Contaminant concentrations in sanctuary habitats do not appear to have the potential to negatively affect living resources or water quality, and conditions do not appear to be changing. Results of a baseline characterization of benthic communities and sediment quality conducted in 2000 (Hyland et al. 2006, Cooksey et al. 2004) suggested that chemical contaminants in sediments (including pesticides, PCBs, PAHs, and metals) were generally at low background concentrations, below probable bioeffect threshold levels. The historically low sediment contamination is most likely attributable to the remote location of this offshore environment and the sandy nature of the substrate (e.g., absence of a silt-clay fraction). However, sediments contain trace concentrations of contaminants associated with human sources (pesticides, PCBs, PAHs), demonstrating that such materials are making their way to the offshore sanctuary environment, either by air or aquatic cross-shelf transport from land (Figure 23). Total organic carbon in sediments is also at low levels — less than 2 percent throughout the sanctuary and less than 1 percent at most stations (Hyland et al. 2006) — typical of shelf waters in this region (Tenore et al. 1978). This is well below a reported range (less than 3.6 percent) associated with a high risk of disturbance from organic over-enrichment (Hyland et al. 2005). Results of a follow-up monitoring survey conducted in 2005 (Balthis et al. 2007) showed a similar persistent trend of low background levels of such sediment-associated stressors. Nonetheless, the presence of chemical contaminants in sediments at low yet detectable levels in both surveys suggests that such pollutants have reached the sanctuary and thus should continue to be monitored to ensure that future problems do not develop (Harris et al. 2004, NMSP 2006).
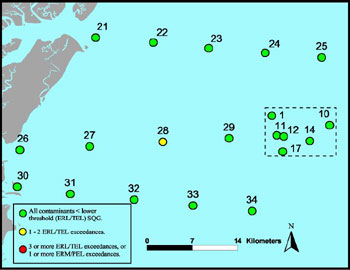 Figure 23. Spring 2001 summary of chemical contaminant concentrations in sediments relative to sediment quality guidelines. The outlined box to the right of the image indicates the Gray's Reef National Marine Sanctuary boundary. (Source: Hyland et al. 2006)
Figure 23. Spring 2001 summary of chemical contaminant concentrations in sediments relative to sediment quality guidelines. The outlined box to the right of the image indicates the Gray's Reef National Marine Sanctuary boundary. (Source: Hyland et al. 2006)
8. What are the levels of human activities that may influence habitat quality and how are they changing?
Selected human activities in the sanctuary have resulted in measurable habitat impacts, but evidence suggests the effects are localized and not widespread. The trend is undetermined. Fishing, anchoring, marine debris, divers and research activities are suspected or known causes of damage to habitats within Gray’s Reef National Marine Sanctuary. Based on boat counts and fishing tournament participation data, visitation to Gray’s Reef has increased over the last 25 years, and this increase is likely responsible for some documented habitat impacts. Anchor damage and entangled fishing line has been observed. The spatial distribution of debris is concentrated in the center of the sanctuary and is most frequently associated with biologically structured habitats (i.e., habitats created by sponges and other upright organisms) and along ledges, rather than at other bottom types. Approximately 90% of debris encountered at the Gray’s Reef sanctuary has been found along ledges (Kendall et al. 2007). This is probably more a result of bottom fishers than tournament fishing (which targets mackerel and involves bait drifting or trolling). Data are not currently available to discern the trend in the number of visitors participating in destructive activities. Nevertheless, continued increases in human use will probably add to habitat alteration. A combination of improved monitoring and enhanced education and enforcement of regulations would be appropriate management actions.
The following information provides an assessment by sanctuary staff and the Gray’s Reef Research Advisory Panel of the status and trends pertaining to the current state of the marine habitat:
Habitat Status and Trends
Status: Good Good/Fair Fair Fair/Poor Poor Undet.
Trends:
▲ Conditions appear to be improving.
- Conditions do not appear to be changing.
▼ Conditions appear to be declining.
? Undeterminted trend.
N/A Question not applicable.
| # |
Status |
Rating |
Basis For Judgement |
Description of Findings |
| 5. |
Abundance/ Distribution |
?
|
Baseline data recently completed; assessment of trends will depend on future observations |
Selected habitat loss or alteration has taken place, precluding full development of living resource assemblages, but it is unlikely to cause substantial or persistent degradation in living resources or water quality. |
| 6. |
Structure |
?
|
Insufficient information on the complex biological structure of habitats to rate condition, though there is evidence of anchor, fishing, and storm damage |
N/A |
| 7. |
Contaminants |

|
Low levels in 2000 and 2005 |
Contaminants do not appear to have the potential to negatively affect living resources or water quality. |
| 8. |
Human Activities |
?
|
Localized within areas of heavy use |
Selected activities have resulted in meaurable habitat impacts, but evidence suggests effects are localized, not widespread. |
Living Resources
Fishes
The highest fish species richness, diversity, abundance and biomass at Gray’s Reef National Marine Sanctuary is found on and near reef structure (‘live bottom’). Resident and non resident reef fishes normally associate with hard structure, and even coastal migratory pelagic species such as mackerels are attracted to and orient themselves near structures. Flat and rippled sand sites have the lowest value in fish species richness, diversity, abundance and biomass. Analysis of fish assemblages at ledges (high-relief hard structure areas) indicates that species richness and total abundance of fish are positively related to total percent cover of sessile invertebrates and ledge height (Kendall et al. 2007). As a result, ledges within the sanctuary are often targeted by fishermen due to the association of recreationally important fish species with this bottom type and because ledges are structurally complex and are often densely colonized by biota. In addition, pelagic predators like king mackerel feed on schools of pelagic baitfish that concentrate down current from bottom structure.
Currently, recreational fishing pressure on reef-associated fishes is thought to be less intense than it is for pelagic species, although studies conducted at the Gray’s Reef sanctuary indicate that fishing mortality for black sea bass is the same or higher within the sanctuary than it is regionally or at inner-shelf reefs off South Carolina (Harris et al. 2005). The most intensive fishing pressure occurs in conjunction with offshore fishing tournaments, which target king mackerel. Weekends experience more fishing activity than weekdays. On an annual basis, fishing pressure is patterned around meteorological events and migratory patterns of the targeted species. Fishing pressure is probably lowest in mid-winter with low temperatures and winter storms. By late winter or early spring, recreational fishing pressure increases as the anglers target black sea bass. In late spring to early summer, fishing pressure peaks as anglers target the pelagic cobia, bluefish, Spanish mackerel and king mackerel. Late summer experiences a slump in fishing pressure as target species are widely scattered and difficult to catch. By fall, fishing pressure increases again as the pelagic species return. This is sustained until the water temperature drops low enough to cause the target species to migrate out of the area.
In 1993, NOAA’s National Marine Fisheries Service’s Marine Resources Monitoring Assessment and Prediction Program established sampling stations at Gray’s Reef sanctuary to monitor reef fish populations. During the trapping periods (July 1993-1995 and July 1998-2001), catches were dominated by black sea bass (50 percent), followed by scup (34 percent) and tomtate (12 percent). Other species caught included pinfish, blue runner, gray triggerfish, northern puffer and leopard toadfish.
In the Gray’s Reef sanctuary, the number of black sea bass caught per trap has increased since 1993, with a significant increase occurring in 2000 (Figure 24). Estimated abundance of black sea bass at the sanctuary showed a large increase from 1993 to 2001, followed by a decrease through 2004. Due in part to a high year of larval recruitment, the population size estimate increased in 2005, and is the second highest estimate since 1993. This species, like many in the snapper-grouper complex, resides on reefs and other structures as adults. Black sea bass are estuarine-dependent as juveniles, and relatively little is known about their spawning behaviors in or near the sanctuary. Tagging data indicated that after three months, 93 percent of the fish were recaptured at Gray’s Reef, suggesting that these fishes show relatively low rates of movement. Tags returns from recreational fishermen outside the boundaries indicate that many of the larger fish move out of the sanctuary (Harris et al. 2004, McGovern et al. 2002).
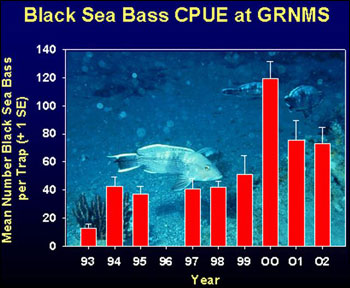 Figure 24. Black sea bass catch at Gray's Reef National Marine Sanctuary through the Marine Monitoring, Assessment and Prediction Program - South Carolina Department of Natural Resources. (Source: Gray's Reef National Marine Sanctuary)
Figure 24. Black sea bass catch at Gray's Reef National Marine Sanctuary through the Marine Monitoring, Assessment and Prediction Program - South Carolina Department of Natural Resources. (Source: Gray's Reef National Marine Sanctuary)
9. What is the status of biodiversity and how is it changing?
Diversity at Gray’s Reef National Marine Sanctuary is very high compared to shelf sites at similar depths north of Cape Hatteras, but there are no baseline data to determine if diversity has changed in response to fishing pressure exerted since the 1970s.
There is considerable benthic, epifauna and fish biodiversity monitoring and data, but data is insufficient at this time to rate the status of the resources and impacts on full community development and function. Benthic infaunal invertebrate diversity in the Gray’s Reef sanctuary is very high, and is higher than comparable depths off mid-Atlantic and northeastern states. The complexity of these structures, however, is not completely understood, and there are no baseline data (i.e., pre-fishing years) to compare with present diversity measures. Diversity of benthic infauna did not change from one study in 2000 (after at least 30 years of commercial and recreational fishing) to a follow-up in 2005 (Cooksey et al. 2004, Hyland et al. 2006). Samples collected had a mean diversity of 45 (+ 11) species per grab (0.04 m2) in 2000 and 47 (+ 12) in 2005. The total number of infauna collected was about 350 taxa. Benthic infauna are an important food source for forage fishes and some fishery species and are an important link in the food chain.
Fish diversity is also quite high in the sanctuary, with 181 species recorded, including 46 managed species (Hare et al. in press). Annual monitoring by visual census has indicated no change in fish diversity (REEF, unpublished). The Gray’s Reef sanctuary is in a transitional zone between cold temperate and warm temperate waters. Because of this, the fish community likely changes considerably in response to episodic hydrographic events like cold water intrusions and Gulf Stream eddies, and may be in a constant state of succession toward full community development.
10. What is the status of environmentally sustainable fishing and how is it changing?
Regional fishing has caused or is likely to cause severe declines in some but not all ecosystem components at Gray’s Reef and reduce ecosystem integrity. Furthermore, this condition appears to be getting worse. According to NOAA’s National Marine Fisheries Service (2006), red snapper, gag, red grouper and black sea bass are overfished. Gray triggerfish, sheepshead and greater amberjack are not currently overfished in the region.
Monitoring of the abundance and size of black sea bass (the dominant reef-associated fishery species at the sanctuary) in trap surveys indicates trends in abundance and size that are similar to trends found throughout the region, where this species is classified as overfished and is undergoing overfishing. This may indicate that federal region-wide fishery management measures have a greater influence on status of stock than do sanctuary regulations. Tagging studies of black sea bass indicate high rates of tag returns from recreational fishermen, resulting from high fishing effort within the sanctuary. Tagging and catch curve analysis from trap survey catches indicate that fishing mortality of black sea bass at the Gray’s Reef sanctuary is as high as or higher than that on other reefs throughout the region. Mean length of black sea bass in trap surveys at the sanctuary has increased since 1993, following similar trends throughout the region, and is likely influenced by increases in minimum size imposed by the South Atlantic Fishery Management Council (Harris et al. 2005). There is good and consistent annual recruitment of small black sea bass in trap catches.
Gag and scamp have decreased in abundance in visual census transects, and length-frequency measurements of black sea bass, gag and scamp (from trap and visual census data) indicate that a large portion of the population is removed upon reaching minimum size, either by fishing or by migration out of the sanctuary.
There is considerable but unmeasured fishing effort on coastal pelagic species (king and Spanish mackerel) during mackerel tournaments and at other times. Federal management of coastal pelagic species has resulted in sustainable fisheries for king mackerel and the stock is not currently overfished.
11. What is the status of non-indigenous species and how is it changing?
The status of non-indigenous species in the sanctuary is considered to be “good/fair” — non-indigenous species exist, precluding full community development and function, but are unlikely to cause substantial or persistent degradation of ecosystem integrity. This trend is declining. Two species of red lionfish (Pterois volitans and P. miles), formerly residents of the western Pacific and eastern Indian oceans only, have become well established in the western Atlantic along the eastern coast of the U.S. (Whitfield et al. 2002) and have been documented at sites in close proximity to the Gray’s Reef sanctuary boundaries. In fall 2007, NOAA’s National Centers for Coastal Ocean Science reported the first sighting of two red lionfish in the sanctuary (Figure 25). Because very few physical characteristics distinguish the two species of red lionfish it is unknown which species was actually sighted. The range and abundance of this species is continuing to increase (Ruiz-Carus 2006). In January 2008, three barnacles of the invasive species Megabalanus coccopoma (titan acorn barnacle) were found in Gray’s Reef attached to the data buoy. These barnacles, native to the western Pacific, have been found throughout the southeast Atlantic.
Potential impacts of these and other organisms include competition with native species for food and space, predation on native species, and diseases to which native species have no resistance (Ruiz-Carus 2006). Impacts from red lionfish could include direct competition with large groupers (Mycteroperca spp.) for food and predation on smaller sea basses (Serranidae spp.) and other benthic fish and crustaceans (Ruiz-Carus 2006). Potential human impacts could result from fishers or divers coming in contact with venomous spines. Impacts from titan barnacles could include spatial dominance of available habitat. Titan acorn barnacles could exclude other epifaunal species, including local barnacles, mussels, oysters, corals and sponges. Cold seasonal water temperatures could hinder year-round establishment of red lionfish (Kimball et al. 2004) and titan acorn barnacles.
 igure 25. One of the two lionfish that were observed for the first time in the sanctuary in fall 2007. (Photo: Matt Kendall/NOAA)
igure 25. One of the two lionfish that were observed for the first time in the sanctuary in fall 2007. (Photo: Matt Kendall/NOAA)
12. What is the status of key species and how is it changing?
The status of key species in the sanctuary is considered to be fair, as selected key species are at reduced levels, but recovery is possible. The condition, however, may be getting worse. Key species of fishes in the sanctuary include gag and scamp, king mackerel, black sea bass and red snapper, all of which are targeted by fishers and are dominant predators in the ecosystem. While gag and scamp can be found at the sanctuary, they are not found in the numbers that might be anticipated based on the abundance of suitable habitat and available resources. Of the 92 ledges surveyed by Kendall et al. (2007), only 20 had occurrences of these species, with the majority of the species occurring on just 10 ledges. The spatial distribution of bothspecies was quite clumped on ledges in the north central and south central regions of the sanctuary. In addition, both species were often observed together at the same ledge and were rarely observed as lone individuals. In contrast, black sea bassoccurred at 98 percent of the ledges surveyed and appeared evenly distributed through-out the sanctuary. Pressure on king mackerel has been steadily increasing at Gray’s Reef in the recent past, with the majority of effort coming from fishing tournaments.
Benthic cover of invertebrates on live-bottom areas in the sanctuary is dominated by various species of sponges (primarily in the genera Ircinia and Chondrilla), corals (predominately Oculina arbuscula), tunicates (including Styela, Aplidium andSymplegma), arborescent bryozoans (primarily Schizoporella) and gorgonians (dominated by Telesto and Leptogorgia) (Ruzicka 2005, Gleason et al. 2007). No evidence of disease has been observed on these key benthic species, although recent mortalities in Ircinia seem to correlate with warmer water temperatures. Recently established long-term monitoring of the benthos has noted some decline in percent cover and species diversity, but these changes appear to be due to storm impacts (i.e., movement of sediment) or represent seasonal cycles (Freeman et al. 2007).
According to NMFS (2008) and SEDAR (2008) , red snapper, gag, red grouper and black sea bass are overfished and/or undergoing overfishing throughout the region. Tagging studies of black sea bass indicate high rates of tag returns from recreational fishermen, resulting from high fishing effort within the sanctuary. Tagging and catch curve analysis from trap survey catches indicate that fishing mortality for black sea bass at the Gray’s Reef sanctuary is as high as or higher than that on other reefs throughout the region. Mean length of black sea bass in trap surveys at the sanctuary has increased since 1993, following similar trends throughout the region, and is likely influenced by increases in minimum size imposed by the South Atlantic Fishery Management Council (Harris et al. 2005). There is good and consistent annual recruitment of small black sea bass in trap catches.
Based on the above information, the status of key species is determined to be fair and the trend appears to be decreasing.
13. What is the condition or health of key species and how is it changing?
The condition of key species at the Gray’s Reef sanctuary has not been systematically assessed, nor have trends been identified. Sponges, recognized as key species at Gray’s Reef due to their importance in structuring habitat, however, have been found to contain organic contaminants (PCBs, PAHs etc.) in their tissues. These filtering organisms appear to be accumulating contaminants from the water column (McFall pers. comm.). Tissues from mussels and fish and sediments have been used recently to determine the level of contaminants in the sanctuary (Cooksey et al. 2004, Hyland at al. 2006), but the amounts present in the sponge tissues appear to be higher than levels reported from these other sources. Coral has also been identified as a key species at Gray’s Reef, with the most prominent species being Oculina arbuscula. This species shows high recruitment rates (Gleason in prep.) and genetic studies indicate that new individuals result from “local” recruitment (Wagner 2006),reflecting a reproductively healthy O. arbusculapopulation in the sanctuary. Insufficient data exist to determine a trend.
14. What are the levels of human activities that may influence living resource quality and how are they changing?
Certain human activities have resulted in measurable living resource impacts, but evidence suggests effects are localized and not widespread. The trend, however, is undetermined. Activities that are most likely to affect living resources at Gray’s Reef are recreational bottom fishing (from boats and perhaps spearfishing), diving (recreational and research), certain research activities (e.g., collecting, coring, data collection), anchoring, disposal of marine debris, and coastal development. Observational data suggest that the activity having the most measurable effect on living resources is recreational bottom fishing. Aside from creating some of the marine debris at Gray’s Reef, fishing appears to depress the size-frequency distribution for black sea bass, potentially affecting their abundance, fecundity and availability as food for other species. Additional information exists to show a regional trend for other species, such as gag and scamp, as well. Existing data suggest that approximately 20 percent of fishers at Gray’s Reef participate in bottom fishing, but time-series data that might be used for assessing trends are not currently available.
Diver impacts, whether they result from research, recreation or spearfishing, are intermittent and generally limited to specific study locations. Similarly, anchoring and marine debris are concentrated in locations with high visitation, and most impacts have been observed in areas with the highest relief and cover. Of the marine debris surveyed at Gray’s Reef, two-thirds is composed of fishing line (usually entangled), which, like other visitation-related activities, is most heavily concentrated in areas of high relief. Data on levels for most of these activities, and for any impacts they might be causing, are generally lacking, as are data on trends.
Preliminary data from one on-going study suggest that evidence of accumulation of certain organic contaminants in sponges likely results from coastal development, but it is not known whether these are at high enough levels to be of concern. Coastal development is certain to continue to increase, making this an activity that should be monitored closely.
The following information provides an assessment by sanctuary staff and the Gray’s Reef Research Advisory Panel of the status and trends pertaining to the current state of the sanctuary’s living resources:
Living Resources and Trends
Status: Good Good/Fair Fair Fair/Poor Poor Undet.
Trends:
▲ Conditions appear to be improving.
- Conditions do not appear to be changing.
▼ Conditions appear to be declining.
? Undeterminted trend.
N/A Question not applicable.
| # |
Status |
Rating |
Basis For Judgement |
Description of Findings |
| 9. |
Biodiversity |
?
|
Considerable benthic, epifauna and fish biodiversity monitoring and data, but insufficient at this time to rate status, trends and impacts as they relate to community development and function |
N/A |
| 10. |
Extracted Species |

|
Black sea bass, gag, red grouper, and red snapper regionally overfished and/or undergoing overfishing |
Extraction has caused or is likely to cause severe declines in some but not all ecosystem components and reduce ecosystem integrity. |
| 11. |
Non-indigenous Species |

|
Two lionfish identified in sanctuary in fall 2007 |
Non-indigenous species exist, precluding full community development and function, but are unlikely to cause substantial or persistent degradation of ecosystem integrity. |
| 12. |
Key Species Status |

|
Removal of key fish species and recent sponge mortality |
The reduced abundance of selected key species may inhibit full community development and function, and may cause measurable but not severe degradation of ecosystem integrity; or selected key species are at reduced levels, but recovery is possible. |
| 13. |
Key Species Condition |
?
|
Key species tentatively identified but unable to determine condition and health; some contaminants detected in sponges, but cause of mortality undetermined |
N/A |
| 14. |
Human Activities |
?
|
Localized within areas of heavy use |
Selected activities have resulted in measurable living resource impacts, but evidence suggests effects are localized, not widespread. |
Maritime Archaeological Resources
There are currently no known shipwrecks in the sanctuary, but Gray’s Reef National Marine Sanctuary does contain considerable paleontological resources of both marine and terrestrial origin. This may have important implications with regard to former human occupation of the area, and the potential for future archaeological finds.
Within the sanctuary boundaries, two research stations have been well documented to contain large areas of ancient scallop (Placopecten magellanicus) beds. These scallops have been dated to approximately 42,000 to 44,000 years before present and range in size up to 20 centimeters in diameter. In addition to the two research stations, the scallops have also been documented at several other sites throughout the sanctuary.
The primary importance of the ancient scallop beds is that they shed some light on past climate change at Gray’s Reef. The presence of these scallops dictates a much colder environment than what is currently found at Gray’s Reef today. The death assemblage also indicates a potentially rapid rise in the ocean temperature at Gray’s Reef that makes the shells proxies for the timing and rate of climate shifts. In the scientific community today, there is great interest in accurate hindcasts for Earth’s climate that provide real context by which to measure and assess modern climate change. These resources may provide a significant role in understanding future climate change, which makes it a vital task to preserve and monitor these “maritime archaeological, paleontological and prehistoric resources” in the sanctuary, as set forth in the enabling legislation of the National Marine Sanctuary System.
Also frequently discovered at the sanctuary are fossilized terrestrial and marine mammal bone fragments. These bone fragments help to piece together the changes at Gray’s Reef as Georgia’s shoreline advanced and retreated over geologic time. It has been well documented that Gray’s Reef was last exposed approximately 7,000 years ago and prior to that had been submerged and exposed many times, allowing both marine and terrestrial animals to live there.
A recent discovery near Gray’s Reef, dated by radiocarbon methods to 38,000 years ago, is that of a northern right whale (Eubalaena glacialis) cranial bone. This discovery alone suggests that the Georgia Bight was a favored ground for this endangered species long before the modern era.
To date, only a few manmade prehistoric artifacts have been recovered at Gray’s Reef. These artifacts have been points of interest and discussion over the years, but no archaeological sites have been discovered in association with these finds. However, since Gray’s Reef was last exposed approximately 7,000 years ago, it is possible that humans once lived and hunted in the area before submergence in post-glacial time (Holocene). It thus remains a possibility that important undiscovered archaeological evidence exists at Gray’s Reef.
Natural oceanographic forces pose the main danger to the Gray’s Reef sanctuary’s prehistoric, archaeological and paleontological resources. Erosion due to storms and natural currents continuously occurs at the bottom, as moving sand exposes and buries the scallop beds and bone fragments. Little can be done to prevent damage to sanctuary resources from these forces except monitor the sites and recover and document any finds as they become exposed. Recreational diving and anchoring at the sanctuary could potentially impact the resources, but since anchoring has been banned within the sanctuary, it is not expected that this will be a major problem.
The following information provides an assessment by sanctuary staff and the Gray’s Reef Research Advisory Panel of the status and trends pertaining to the current state of the sanctuary’s maritime archaeological resources:
Maritime Archaeological Resources and Trends
Status: Good Good/Fair Fair Fair/Poor Poor Undet.
Trends:
▲ Conditions appear to be improving.
- Conditions do not appear to be changing.
▼ Conditions appear to be declining.
? Undeterminted trend.
N/A Question not applicable.
| # |
Status |
Rating |
Basis For Judgement |
Description of Findings |
| 15. |
Integrity |
N/A
|
No archaeological evidence, though former human occupation remains a possibility based on paleontological data |
N/A |
| 16. |
Threat to Environment |
N/A
|
No archaeological evidence, though former human occupation remains a possibility based on paleontological data |
N/A |
| 17. |
Human Activities |

|
Potential for diving, fishing and anchoring to damage sites |
Some potentially relevant activites exist, but they do not appear to have had a negative effect on maritime archaeological resource integrity. |